Energetic materials
The synthesis of energetic, non-nuclear materials for military and space application has been a long term goal in our research group. Some of the current challenges that face energetic material scientists are:
- Different target types: Tunnels and caves have become more important as the worldwide war against terrorism tracks groups such as Al Qaeda to the remote areas of the Mid-East.
- Time-critical targets: Rapid response to attack the target before it moves is critical.
- Minimization of unwanted effects: Low Collateral Damage Munitions, i.e. munitions that cause little damage aside from damage to the intended target, have become more important as sites of military interest are increasingly co-located with civilian population centers.
- Continuing interest in insensitivity: Insensitive Munitions for reduced vulnerability of munition stores and increased survivability of munitions that are subjected to very stressful conditions under their normal usage are important from an economic standpoint, but even more so from the point of view of lives saved and military missions completed.
In general, the survivability increases with:
- low observable plumes (Fig. 1)
- ballistic protection
- long-range
- deep-targeting
- early attack
- first round kill


Fig. 1. Left: conventional (AP based) formulation; Right: smokeless (HZT (1) + ADN) combustion.
Modern high-energy-density materials (HEDM) derive most of their energy either
- from oxidation of the carbon backbone, as with traditional energetic materials or
- from their very high positive heat of formation. Examples for the first class are traditional explosives such as TNT, RDX and HMX.
Modern nitro compounds such as CL-20 or the recently reported hepta- and octanitrocubanes possess very high densities and enhance the energies utilizing substantial cage strain. Members of the second class of compounds are 5,5’-azotetrazolate salts which show the desired remarkable insensitivity to electrostatic discharge, friction and impact while having a very high heat of formation (Fig. 2, 3).
Our research group developed the synthesis and characterization of
- [N2H5]+2[N4C-N=N-CN4]2- (1, HZT), [N2H5]+2[N4C-N=N-CN4]2- · 2 H2O (2) and
- [N2H5]+2[N4C-N=N-CN4]2- · 2 N2H4 (3),
which are new members of the second family which derive most of their energy from their very high positive heats of formation (Fig. 2 and 3).
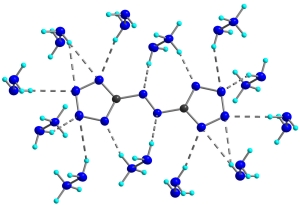
Fig. 2. Structures dihydrazinium 5,5’-azotetrazolate dihydrazinate (3).


Fig. 3. Steel sleeve (Koenen test) (1, HZT).
Several approaches are being pursued to provide new energetic materials to meet the challenges of the future. One approach is the synthesis of all-nitrogen or nitrogen-rich energetic materials. Support for work in this area has been provided by the German Federal Office for Defense Technology and Procurement (BWB), the Bundeswehr Research Institute for Materials, Fuels and Lubricants (WIWEB), the US Army Research Laboratory (ARL), the European Research Office (ERO) and the US Navy (NSWC, Indian Head).
The expected advantages of nitrogen-rich and all nitrogen compounds include:
- all gaseous products
- high heats of formation
- high propulsive/explosive power
- high specific impulse (>200% hydrazine)
- very high flame temperatures (7500 K).
It is still too early in the development of such materials to assess whether they will live up to their potential, but nonetheless they represent a very exciting and challenging new area of chemistry.
Recent modeling has shown that the presence of high concentrations of nitrogen species in the combustion products of propellants can reduce gun barrel erosion by promoting the formation of iron nitride rather than iron carbide on the interior surface of the barrel. Thus, compounds such as guanidinium azotetrazolate (C4H12N16, see above, cf. Fig. 2) show promise for use in low erosivity gun propellants.
Other highly energetic compounds which were developed in our research group at LMU include:
nitriminotetrazole triaminoguanidinium nitroformate
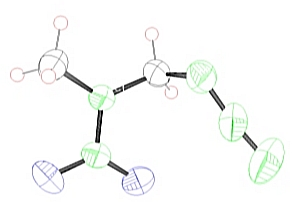
hydrazinium azide (N>90%) 1-azido-2-nitro-2-azapropane
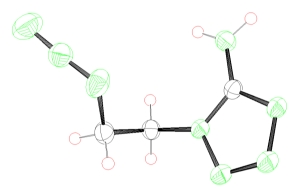
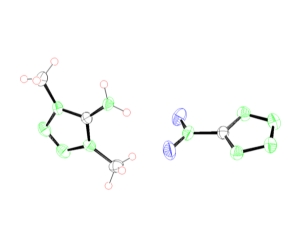
azidoethyl aminotetrazole tetrazolium nitrotetrazolate

